2.2 Equilibrium and Yield
Assumed knowledge
Equilibrium graphs
Chemical systems may be open or closed.
Over time, reversible chemical reactions carried out in a closed system at fixed temperature eventually reach a state of chemical equilibrium.
The changes in concentrations of reactants and products, as a system reaches equilibrium, can be represented graphically.
- Draw and interpret graphs representing changes in concentrations of reactants and products.
Video: What is Dynamic Equilibrium?
Video: Rates and Equilibrium (especially chapter 6)
Kc calculations
The position of equilibrium in a chemical system at a given temperature can be indicated by a constant, Kc, related to the concentrations of reactants and products.
- Write Kc expressions that correspond to given reaction equations for homogeneous equilibrium systems.
- Undertake calculations involving Kc and initial and/or equilibrium quantities of reactants and products for homogeneous equilibrium systems.
Videos: The Concepts | Applying the Concepts
Le Châtelier’s principle
The final equilibrium concentrations, and hence position of equilibrium, for a given reaction depend on various factors.
- Predict and explain, using Le Châtelier’s principle, the effect on the equilibrium position of a system of a change in the:
- concentration of a reactant or product
- overall pressure of a gaseous mixture
- temperature of an equilibrium mixture for which the
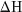 value for the forward or back reaction is specified.
value for the forward or back reaction is specified.
- Predict the change that occurred in a system, or whether a reaction is exothermic or endothermic, given the effect of the change on the equilibrium position of the system.
